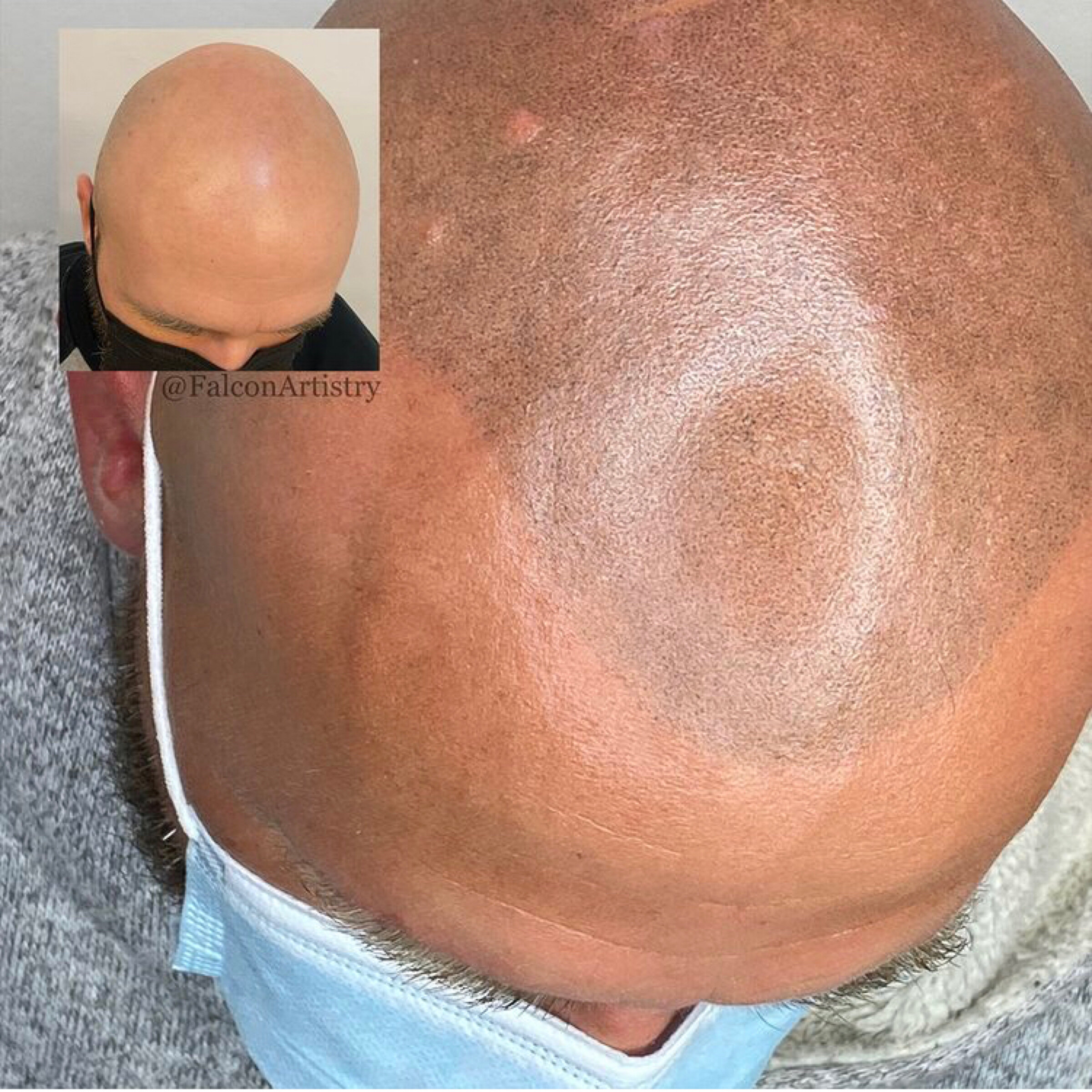
Finasteride inhibits 5-alpha reductase, the enzyme that is responsible for converting testosterone to dihydrotestosterone (DHT) and that is present in higher concentrations in and around the hair follicles of individuals with androgenetic alopecia.
Most people who use finasteride have a rapid decrease in serum DHT concentration, which stops or slows the miniaturization of affected hair follicles in men with androgenetic alopecia. Women typically require a higher dose than men to achieve a benefit to their hair.
Finasteride is one of three FDA approved treatments for androgenetic alopecia in men and is sometimes prescribed off-label for postmenopausal women (or those with no plans of getting pregnant). Low risks of sexual side effects, mood changes and other undesirable side effects have prompted more research into the use of topical finasteride.
Recent studies have indicated that topical finasteride significantly decreases the rate of hair loss, improves hair count compared to placebo, and could be just as effective at treating AGA as oral finasteride. While it appears to be well-tolerated, it is important to note that despite lower systemic exposure, it still significantly supresses serum DHT levels. Because of this, the possibility of side effects similar to oral finasteride still exist, but the risk is thought to be lower.
Overall, it seems that topical antiandrogens are worth further investigation and may be worth considering before oral versions for those with AGA.